Choose language:
KIDNEYS
Kidneys are bean-shaped paired organs, about 10–12 cm long (fist-sized) and together weigh about 300 g. An individual usually has two kidneys – one on each side of the spine, in the back part of the abdomine in the region of the lower ribs.
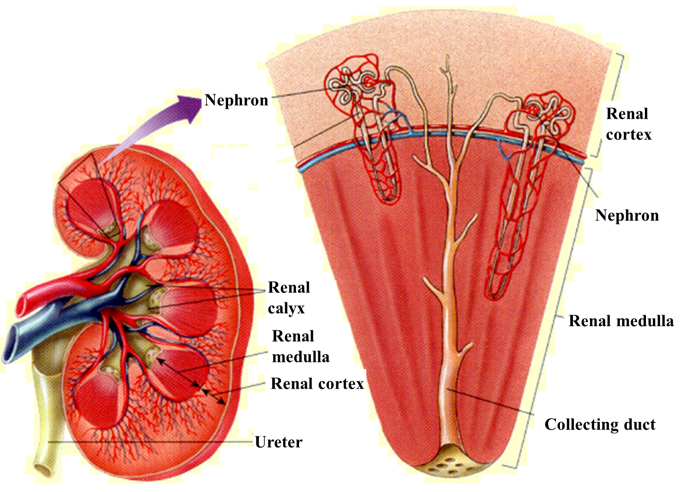
The purpose of the kidneys is to remove waste products and excess fluid from the body as urine. In addition, the kidneys play an important role in blood pressure regulation, maintaining the body’s acid-base balance and regulation of calcium and phosphorus metabolism. The kidneys also produce erythropoietin, a hormone that has an important role in the formation of blood cellular components.
Each kidney contains about 1.2 million nephrons. A nephron is the main unit of kidney function. Each nephron is a functional unit able to perform specific transport functions. Only nephrons can manage the specific task of urine production. The damage of a large number of nephrons causes kidney function disorders.
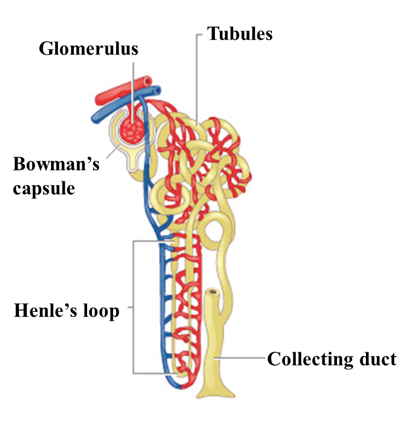
The nephron comprises of a renal corpuscle in the renal cortex, consisting of a cluster of capillaries called a glomerulus and an encompassing glomerular capsule (Bowman’s capsule).
Renal tubules are the main part of the nephron. The tubules are a thin-walled tubule extending from the glomerular capsule. [1]
KIDNEY DISEASE AND DIALYSIS
Most often, kidney disease damages both kidneys at the same time. As a result of the disease, kidney (renal) failure may develop. This is a condition where kidney function declines or ceases. In this case, kidneys are no longer able to clear the blood of waste products or regulate fluid in the body. For the body to function adequately, alternative renal replacement therapy is required. Renal replacement therapy includes haemodialysis (blood dialysis), peritoneal dialysis, and renal transplantation. [2] The information supplied here focuses primarily on haemodialysis
Dialysis is a treatment method for end-stage (chronic) renal disease that is used when the kidneys are not able to clear the body of waste products formed as a result of metabolism or excrete urine. Dialysis therapy is required when 90% or more of normal kidney function is lost – this may be caused by acute kidney damage, or chronic kidney disease developing over several years. [3]
In the case of end-stage kidney disease, it is recommended to start preparing for renal replacement therapy while the patient is still feeling well, but according to blood and urine analyses, the kidneys are not able to keep up with the body’s metabolic needs any more. The symptoms of chronic kidney disease are nausea, poor appetite, fatigue, vomiting, concentration difficulties, but with the timely initiation of renal replacement therapy, these conditions diminish. [4]
METABOLIC WASTE PRODUCTS
Chronic kidney disease does not allow the body to excrete waste products from metabolism. These waste products, the accumulation of which causes disruption of the normal functioning of the body, are called uremic toxins. Currently, about 90 different uremic toxins are known.
The aim of renal replacement therapy (haemodialysis, peritoneal dialysis or kidney transplantation) is to reduce the amount of uremic toxins in the body and to avoid damage associated with the accumulation of waste products.
The uremic toxins are classified on the basis of molecular weight: large, medium, and small molecules. There are molecules bound to proteins and it has not been possible to measure their removal during haemodialysis until now [5] when a new technology for on-line monitoring of uremic toxins is under development. [https://cop.optofluidtech.com]
WHAT IS HAEMODIALYSIS?
Haemodialysis is a procedure during which chemical balance is mostly restored in the body and waste products and excess fluid are removed.
The procedure is performed with a dialysis machine. The role of kidneys is performed by a special filter – dialyser. During haemodialysis, blood is directed through a tube system (dialysis blood line) to the dialyser, where waste products and excess fluid, if necessary, are removed from the blood. The cleaned blood is then reintroduced back into the body. For the blood to reach the tubes and dialyser, a vascular surgeon must create a fistula – a special connection between an artery and a vein. Sometimes, when it is not possible to create a fistula, a dialysis catheter is introduced into a large blood vessel (into the neck or a femoral vein). [6]
During the procedure, an anticoagulant (medicine that prevents blood clotting) is injected into the machine so that blood will not clot while outside the body in the dialysis machine.
During the haemodialysis procedure, blood is constantly circulating, moving from the body through the dialyser and back. The more times blood passes through the dialyser, the cleaner it becomes. During one dialysis treatment, 60–100 litres of blood passes through the dialyser, which means that the entire volume of blood in the body passes through the machine 10–20 times.
The haemodialysis procedure is usually performed at a hospital or dialysis unit. In the case of certain conditions and training, it may possible to perform haemodialysis regularly at home.
WHEN IS HAEMODIALYSIS REQUIRED?
Haemodialysis is usually performed when the kidneys do not work sufficiently and effectively to meet the body’s needs. In this case, waste products accumulate in the blood, little or no urine is produced, and/or excess fluid (oedema) may be present. Such a condition may be temporary (acute kidney failure) or permanent (chronic kidney disease).
In chronic kidney disease, about 90% of kidney function has been lost.
Both blood and urine analysis are used to evaluate kidney function.
HOW DOES BLOOD GET TO THE DIALYSER?
To perform haemodialysis, blood must be directed from the patient through tubes and dialyser and back to the patient. To ensure a sufficient volume of blood for dialysis machine operation, a dialysis access fistula needs to be created before commencing haemodialysis. A fistula is a connection between an artery and a vein created by a vascular surgeon, where dialysis cannulas/needles are inserted before each procedure. At the beginning of each dialysis treatment, two needles are inserted into the fistula: through one, blood is directed to the dialyser, away from the body, and through the other, cleaned blood is returned to the body. In some cases, it is not possible to create a fistula, and the procedure is performed with the help of a dialysis catheter inserted in a major blood vessel. The dialysis catheter is attached to to the skin to avoid shifting.
WHAT IS A FISTULA?
A fistula is a special connection between two blood vessels, a vein and an artery, created by a vascular surgeon. Usually, it is situated on the patient’s non-dominant forearm, but much depends on the condition of the blood vessels and patient’s anatomy.
Sometimes, a special artificial vessel, also called a graft or prosthetic fistula, is placed under the skin, to create the connection.
After surgery, the fistula needs about six weeks to heal before it can be used for haemodialysis.
WHAT IS A CENTRAL VENOUS DIALYSIS CATHETER?
A central venous dialysis catheter is a catheter with a diameter of several millimetres that is introduced into a large vein, usually a jugular vein (in the neck), femoral vein (in the groin), or subclavian vein (under the collarbone). Typically, a central venous dialysis catheter has two branches – from one, blood is directed to the haemodialysis machine and through the second, cleaned blood is transferred back to the body. The catheter is fixed with sutures (stitches) to protect it from moving. The area where the catheter is placed under the skin is called the exit-site. A central venous dialysis catheter can be used for haemodialysis immediately after placement; therefore, its placement may be required before permanent access has been created or a fistula has matured.
WHICH IS BETTER: FISTULA OR CENTRAIL VENOUS DIALYSIS CATHETER?
A fistula is preferred, as its life duration is usually longer, and the risk of infection is considerably lower. Central venous dialysis catheters have an increased risk of infection in both the catheter and the exit-site region. Scar tissue and blood vessel stenosis may develop in blood vessels after catheter placement. The average duration of central venous dialysis catheters is two years, after which they should be replaced. Additionally, a fistula allows more effective haemodialysis than a central venous dialysis catheter, as higher blood flow rates are often achieved. [7]
HOW TO AVOID INFECTION RISK WITH CENTRAL VENOUS DIALYSIS CATHETER?
After the placement of a central venous dialysis catheter, it is important to monitor that the dressing covering the exit-site is always dry, clean and intact – one cannot take a bath or go swimming with it. When showering, the exit-site should be protected from becoming wet as much as possible. A wet patch should be immediately replaced. Note, that wet on the outside and still intact is harmless.
In the case of fever, chills and tenderness over the exit-site, it is important to inform the haemodialysis unit.
The central venous dialysis catheter plugs/caps must not be removed at home. If arm or face swelling or haemorrhage suddenly occurs on a haemodialysis-free day, it is important to inform the haemodialysis unit and/or renal doctor.
Sometimes, blood clots (coagulates) in the central venous dialysis catheter and, in this case, the clot (thrombus) should be dissolved with a special medicine – it requires time to work; so more time may be spent in the haemodialysis unit. If coagulation repeatedly occurs, the haemodialysis catheter may be replaced. [7]
WHAT PATIENTS SHOULD KNOW ABOUT HAEMODIALYSIS?
The patient should know the changeable variables of his/her individual haemodialysis:
- the blood flow rate
- if potassium or calcium replacement needed during haemodialysis
- the duration of dialysis
- anticoagulant dose.
The individual characteristics of the patient’s dialysis therapy are presented on the patient’s monitoring sheet (prescription) and these are changed in accordance with the patient’s condition.
WHAT IS EFFECTIVE HAEMODIALYSIS?
Effective haemodialysis is haemodialysis that ensures the optimal level of waste product removal, corrects metabolic shifts, and reduces fluid volume. The aim of the procedure is to improve how the patient is feeling and to support the body’s function.
There are three important factors for haemodialysis adjustment:
- treatment duration – the standard duration of haemodialysis is 240 min (4 hours) three times a week; usually every other day. Healthy kidneys work 24/7, but the duration of haemodialysis is, on average just 12 hours a week – only half a day! Therefore, as with healthy kidneys where waste products are corrected to normal levels, this cannot be achieved with haemodialysis. However, a stabilisation of waste products can be achieved with an adequate dialysis regimen.
- Blood flow rate – the faster the movement of blood through the dialysis machine, the more times it passes through the dialyser and the cleaner it becomes. Fistulas are the preferred as dialysis access, as they allow a higher blood flow rate than central venous dialysis catheters, ensuring more effective cleaning of the blood.
- Dialyser surface – dialysers with a larger surface area clean more effectively. But it should be taken into account that larger filters also need a higher blood flow rate for the blood to not coagulate in the filter.
HOW TO ASSESS THAT HAEMODIALYSIS IS EFFICIENT AND EFFECTIVE?
One aim of dialysis therapy is to clear the body of excess waste products due to metabolism. The level of waste products in the body, as well as achieving the metabolic balance, can be assessed on the basis of blood analyses. It is possible to calculate the relative reduction of waste products (i.e. comparing the amount before and after haemodialysis) and evaluate whether the blood is sufficiently cleaned after the procedure. This relative reduction is measured via Urea Reduction Ratio (URR) or the level of urea cleaned from the blood during a dialysis treatment and it is measured in percentage. Urea is one of the waste products of metabolism and URR is calculated via blood analyses.
In a patient receiving haemodialysis procedures, the removal of urea during haemodialysis can be calculated. URR should be optimally over 70% (minimal acceptable level 65%) and Kt/V over 1.4 (minimal acceptable level 1.2). Dialysis therapy should be adjusted if required, so that the removal of waste products would be more effective. In the case of higher clearance values, the reduction of dialysis duration is not required – a better dialysis is in the best interest of the patient. Sometimes, when the treatment target (URR under 65% or Kt/V under 1.2) cannot be achieved, dialysis duration or frequency should be increased, and perhaps additional treatments performed.
A regular high-quality and patient-individualised haemodialysis treatment improves patient’s well-being and general condition. At the commencement of renal replacement therapy, when the changes of waste products before and after the procedure are very noticeable and the body is not able to adjust, the patient may feel worse despite adequate haemodialysis. How the patient feels during and after dialysis is also influenced by the removal of large volume of fluid, due to changes in cardiovascular function. Usually, the problems are transient, and it is important to discuss them with the renal doctor. Studies have shown that effective haemodialysis prolongs life, improves the patient’s health condition, and avoids complications accompanying chronic kidney disease.
DIALYSIS DURATION – CAN IT BE DONE FASTER?
Dialysis duration and frequency (dialysis dose) is determined by the renal doctor for each individual patient according to the patient’s needs. It depends on the following circumstances:
– how much the patient’s own renal function has been preserved;
– how much weight gain due to fluid accumulation between dialyses;
– how high is the concentration of waste products in the blood;
– how high is the concentration of potassium and phosphorus in blood;
– the highest blood flow rate that can be achieved during dialysis (i.e. how well the dialysis access works).
The dose may change over the course of time. Generally, as the function of the patients own kidneys diminishes, the dialysis dose may be increased.
Already when starting dialysis, all patients must be ready and agree that a dialysis procedure lasts 4 hours and the procedure occurs 3 times a week. For a patient with very low urine production, 12 hours of dialysis a week is the minimum to ensure sufficient metabolic support for clearing waste products from the blood.
Nonetheless, it should be kept in mind that such a dialysis dose replaces only about 7% of healthy kidney function. Therefore, it is very important that the dialysis procedure is as effective as possible. When haemodialysis is less effective, the treatment must be intensified – the procedure duration should be lengthened or procedure frequency increased. Better dialysis helps to maintain or even improve the patient’s general condition.
WHY IS BLOOD FLOW RATE IMPORTANT?
Blood flow rate is an important factor in changing the efficacy of haemodialysis; this allows greater cleaning of the blood – blood passes through the filter several times and the content of waste products is lower when the haemodialysis treatment is finished. Therefore, a blood flow rate that is as fast as possible is a factor in effective treatment. Generally, 350 ml/min is considered sufficient in patients with fistula, and it can be up to 450 ml/min, but this necessitates the use of needles with a larger diameter.
WHAT IS A DIALYSATE?
During dialysis, blood passes repeatedly through a special filter – a dialyser. A special dialysis fluid (dialysate) that is separated from the blood by semipermeable membrane, also flows through the dialyser. Semipermeable membrane is a membrane that allows some particles to pass through (for example by size). During cleaning, waste products and excess fluid are directed through the membrane from blood to the dialysis fluid and then down the drain. The dialysate flow rate should be at least 1.5 times the blood flow rate and is usually regulated automatically by the machine.
CHOICE OF FILTER AND DIALYSER
Various filters (dialysers) are membranes with a different surface area chosen depending on the patient – smaller patients achieve effective haemodialysis with filters with a smaller surface area, larger patients usually requiring larger filters.
During the initial haemodialysis treatment, a filter with a smaller surface area is chosen because a rapid removal of waste products could make the patient feel worse. In addition to weight/height, blood flow rate and dialysate flow rate should also be taken into account to use the membrane surface area in the dialyser to the maximum extent. Maximum parameters are determined for every filter by the manufacturer.
WHEN SHOULD RENAL REPLACEMENT THERAPY BE PREPARED? WHY IS IT NEEDED?
The preparation for renal replacement therapy includes several aspects including that it is important for the patients understanding of the disease course and to make changes to the way of life. A planned start to renal replacement therapy allows avoiding the life-threatening accumulation of waste products and the development of complications due to chronic kidney disease. Usually, the patient tolerates a scheduled dialysis therapy better than an irregular one. Renal replacement therapy should be considered when the severity of the patient’s chronic kidney disease is G4/stage 4 (eGFR less than 30 mL/min/1.73 m2). Nonetheless, even at this stage, it may take years for the patient to actually get to commence renal replacement therapy. It is possible to slow the development of renal failure and thus delay the start of renal replacement therapy with a correct diet and medicine. The kidney doctor (nephrologist) should be visited regularly to evaluate the stability or deterioration of renal failure and to start treatment preparation in time. For example, the creation of fistula should be planned at least 6 weeks before the start of dialysis, so that the fistula should be ready.
WHAT ARE COMMON PROBLEMS DURING HAEMODIALYSIS?
- Any problems arising during haemodialysis are evaluated and treated by the renal unit staff, and may include:
- a low blood pressure during dialysis or after the procedure – usually associated with an excess volume of fluid in the body or a fluid overload before dialysis and the need to remove a large volume of fluid during dialysis;
- muscle cramps – associated with a low calcium level or the excess removal of fluid; this can be assessed with the help of a target weight (dry weight);
- nausea and vomiting – may occur at the beginning of renal replacement therapy; disappears when waste products have reached a level where metabolism has stabilised;
- headache – may occur due to a rapid change of blood waste product level; more commonly associated with high blood pressure.
ARE TEST RESULTS COMPARABLE TO PEOPLE WITHOUT CHRONIC KIDNEY DISEASE?
Regrettably not; completely normal blood and urine analyses would be observed if haemodialysis functioned like our own kidneys – 24 hours a day. However, with the help of renal replacement therapy, it is possible to keep the amount of waste products stable and makes it possible for the body to perform almost normally:
Blood analyses are done once every month (more frequently if needed) and treatment – both medication treatment and haemodialysis treatment – is adjusted accordingly.
Pre-dialysis potassium should be 4.0–5.5 mmol/L.
Haemoglobin value should be in the range of 105–115 g/L.
Each patient requires an individual treatment plan due to his/her disease and health condition and therefore, blood and urine analysis results and treatment changes are discussed with the patient. It can take a while to feel relatively well again, as adjusting to dialysis treatment is different for everyone.
REFERENCES
1. Jana Jerotskaja “Dialüsaadi UV-kiirguse sumbuvusspektrite ja dialüüsil elimineeritavate ainete vahelise seose analüüs”, Tallinn 2007
2. Kroonilise neeruhaiguse patsiendijuhend (PJ-N/20.1-2017) https://www.ravijuhend.ee/patsiendivarav/juhendid/71/kroonilise-neeruhaiguse-patsiendijuhend
3. https://www.uptodate.com/contents/hemodialysis-beyond-the-basics#H1
5. https://www.uptodate.com/contents/uremic-toxins
7. https://www.kidney.ca/document.doc?id=764
8. J. Levy, J. Morgan ja E. Brown, 2001; NK DOQI quidelines; National Kidney and Urologic Diseases Information Clearinghouse. Fresenius Medical Care study material
9. Daugirdas JT (1995) Simplified equations for monitoring Kt/V, PCRn, eKt/V, and ePCRn. Adv Ren Replace Ther.; 2(4):295-304.
10. Daugirdas JT, Depner TA, Gotch FA, et al (1997) Comparison of methods to predict equilibrated Kt/V in the HEMO Pilot Study. Kid-ney Int.; 52(5):1395-1405.
Useful links



